-
Revisions to DOH Blood Lead Testing Recommendations
01/26/2026 The Washington State Department of Health (DOH) has revised the state’s blood lead action level to 3.5 µg/dL, to be more protective of children’s health and prevent further exposure. DOH recommends that health care providers begin blood lead confirmatory and follow-up testing at 3.5 µg/dL. The Notifiable Conditions rule, which requires all blood…
-
Adult Syphilis Treatment Guidelines
This article is excerpted from CDC’s 2015 STD Treatment Guidelines. The preferred drug for all stages of syphilis treatment is Penicillin G administered parenterally. Preparation, dosage, and length of treatment depend on the stage and clinical manifestations of the disease. Selection of the appropriate penicillin preparation is important. T. pallidum can reside in sequestered sites…
-
Flu Patient Information
Worried about the flu? Influenza is widespread and at epidemic levels. The best way to protect yourself and your family is still getting a flu vaccine. Frequent hand washing and avoiding others who are sick also helps. Flu vaccines can take up to two weeks to take effect. What if, despite your best efforts, you…
-
Tuberculosis Screening
Consider testing Person traveling in TB endemic areas. Migrant workers. Person experiencing homelessness. Contact to a tuberculosis case. Foreign-born person from tuberculosis-endemic area. Person who injects drugs. Immunosupressed person (e.g., HIV positive or organ transplant). Resident/employee of healthcare, correctional or long-term care facility. Person with chronic medical problem (e.g., diabetes, end stage renal disease). Test…
-
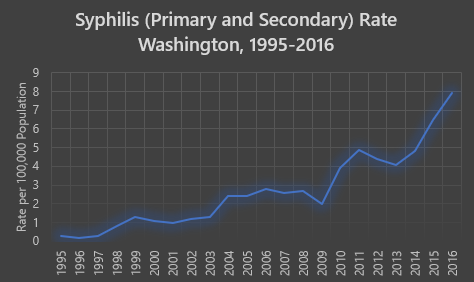
Health Advisory: Syphilis Increase and Prevention of Congenital Syphilis
Requested Actions Test all pregnant women for syphilis at the first prenatal encounter. Repeat syphilis testing during the third trimester among women at risk for STD (e.g., recent history of bacterial STD, multiple partners, homelessness; methamphetamine, opioid, or cocaine use; exchanging sex for money, drugs, etc.; having a sex partner who is a man who…
-
Hepatitis B Immunization and Healthcare Workers
Healthcare Worker Immunization Pre-exposure evaluation for healthcare personnel previously vaccinated with complete, ≥ three dose hepatitis B vaccine series who have not had post-vaccination serologic testing* Source: CDC—MMWR December 20, 2013. * Should be performed one to two months after the last dose of vaccine using a quantitative method that allows detection of the protective…
-
Latent Tuberculosis Infection Treatment Guidelines
High-Priority Candidates for Latent Treatment Infection (LTBI) Treatment Positive QuantiFERON-TB Gold (QFT) (>0.35 IU) Tuberculosis Skin Test (TST) ≥5 mm HIV-positive people. Recent contacts of person with infectious tuberculosis (TB). People with fibrotic changes on chest x-ray (CXR) suggestive of previous TB; or inadequate treatment. People with organ transplants or immunosuppression therapy. TST ≥10 mm…
-
Infection Control Guidelines
Standard Precaution When to Use Standard Precautions During all patient encounters—prevents the spread of bloodborne pathogens such as HIV, Hepatitis B and Hepatitis C. Reason to Use Standard Precautions Protects the healthcare worker from patient’s potentially contaminated body fluids and prevents the spread of disease to others. Components of Standard Precautions Wash hands before and…
-
Controlling Norovirus
You may hear norovirus called “the stomach flu.” Some viral illnesses that cause vomiting and diarrhea are caused by norovirus. You can become infected with norovirus many times in your life. Norovirus Is very contagious. Norovirus can spread quickly in places like daycare centers, nursing homes, schools, restaurants and cruise ships. Causes diarrhea and vomiting,…
-
Tdap and Pregnancy
Pertussis is a serious disease for young infants. Pertussis epidemics occur in the United States every three to five years. Even though we have a vaccine, pertussis is a common infectious disease. There are 10,000 to 40,000 cases and 10 to 20 deaths per year.[1] Pertussis is most serious in infants younger than six months.…
-
Chlamydia Diagnosis Follow-up
Provider Checklist Patient treatment for chlamydia is not complete without partner treatment. After receiving a positive chlamydia result, check the following steps to help stop the spread of chlamydia and to protect your patient from reinfection: Provide patient education on essential points: Take medications as prescribed. Do not have sex for seven days after treatment,…
-
Hand Hygiene In Healthcare Settings
What is hand hygiene? Hand hygiene refers to the use of hand washing with soap and water or alcohol hand sanitizer (60% alcohol or greater) in order to reduce infection rates, reduce transmission of antimicrobial resistant organisms and stop outbreaks of communicable disease. Why is hand hygiene important? When should I use hand hygiene? How…
-
Environmental Cleaning
Quick Reference: Environmental Cleaning How to clean equipment and surfaces, when to clean, what to use Visibly Soiled surfaces: Scrub the surface with a cleaner/detergent or a disinfectant that contains a cleaner/detergent. Wear gloves! May need to rinse (check label). Will NOT need to rinse if using a wipe that contains both a detergent and…